Eliza Stroh, MS, CGC, Cord Blood Clinical Specialist

This July, Insception Lifebank highlights the triumphs of newborn stem cell science as we continue to spread the word about the valuable resource of cord blood and cord tissue preservation. Here, we review the unique properties of these remarkable cells, celebrate the successes of stem cell medicine, and reiterate the potential of newborn stem cells in future applications.
The fundamental role of cord blood in transplant medicine
For more than 30 years, cord blood has served as an increasingly important source of lifesaving stem cells for treatment of certain blood disorders, immune disorders, cancers, and metabolic disorders1 (See figure below). Today, there are over 80 conditions for which cord blood may be used in transplant medicine,2 and more than 45,000 stem cell transplants have used cord blood from public and private family banks.3
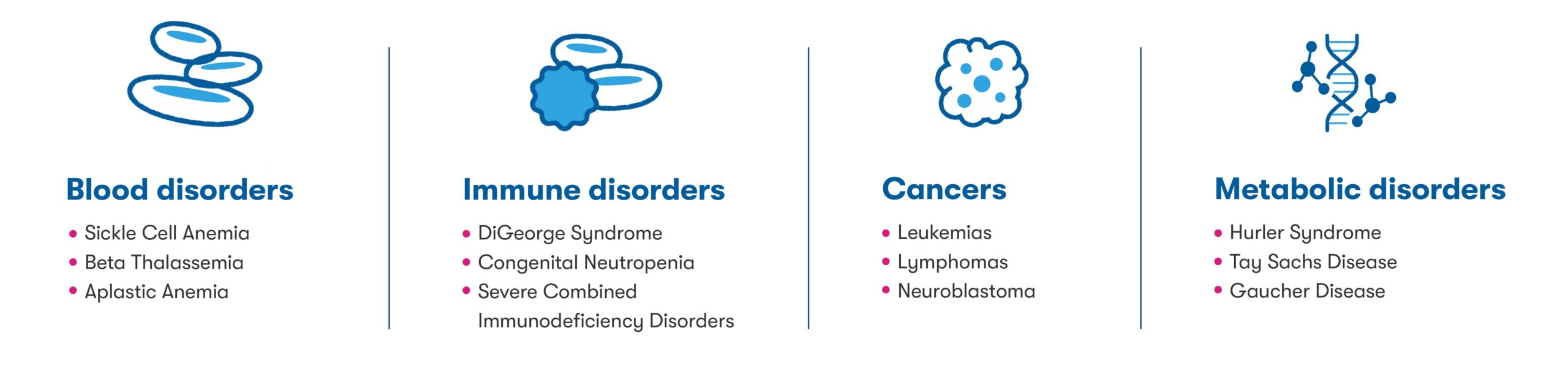
The emergence of cord blood and cord tissue in regenerative medicine
In addition to the proven and established use of cord blood in transplant medicine, many families have used their stored cord blood for investigational applications in the field of regenerative medicine. Years of preclinical research has demonstrated the ability of newborn stem cells to migrate to sites of injury, reduce inflammation, and support the repair and regeneration of damaged tissue.4 This research has led to the initiation of over 500 clinical trials worldwide,5 and families with access to their stored newborn stem cells have participated in research for conditions including cerebral palsy, hearing loss, and hydrocephalus6 (See figure below).5,6,7
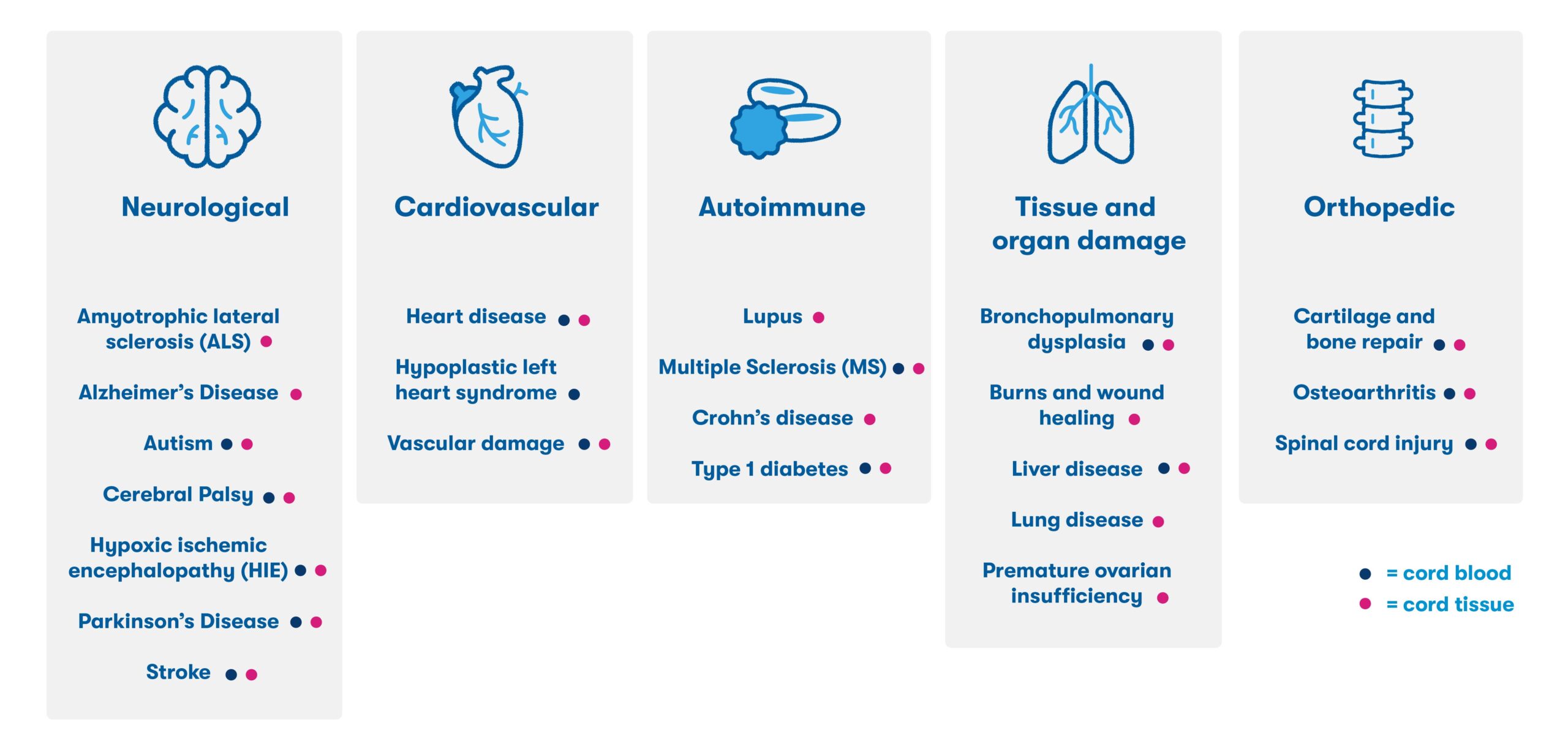
Based on the anti-inflammatory properties of newborn stem cells and their ability to regulate immune dysfunction, future applications of newborn cells may aim to treat age-related degenerative diseases, chronic conditions, and other ailments resulting from long-term inflammation and tissue damage.6 It is estimated that 1 in 3 people may benefit from regenerative therapies in their lifetimes,8 and our hope is that more and more families will have access to their own newborn stem cells through private banking.
Increased matching flexibility means access for more family members
The pristine nature of newborn stem cells makes them more flexible than bone marrow- or peripheral blood-sourced stem cells with respect to matching, and transplants can occur when the donor and recipient share only half of their HLA markers.1 Closer HLA matching leads to better treatment outcomes,9 including decreased risk of certain transplant complications like graft versus host disease.1 Biological parents are a partial match to their children’s cord blood stem cells, and full siblings have a 75% chance of being a sufficient HLA match.
Newborn cells for applications in innovative medicine: Immunotherapy and gene therapy
Newborn stem cells are already being used in research as a source of material for ground-breaking treatments in immunotherapy, which aims to restore or enhance the immune system’s response to diseases. One type of immunotherapy utilizing cell types found in cord blood harnesses and amplifies the natural anti-tumor effects of NK (natural killer) and T cells using molecules known as chimeric antigen receptors, or CARs. CAR-NK and CAR-T therapies have revolutionized cancer treatment10 and newborn stem cells are an ideal source of cells for these therapies since they are young, healthy cells.11,12Additionally, regulatory T cells from cord blood could also potentially revolutionize the treatment of autoimmune disease.9
As the emerging field of gene therapy moves to revolutionize the treatment of inborn conditions, newborn stem cells may also prove to be an ideal source of cells for ex vivo manipulation.13 By taking advantage of the one-time opportunity to preserve pristine newborn stem cells for future use, parents are setting aside a resource that may extend to the most advanced of future medical technologies.
Delayed cord clamping (DCC) is not an impediment to cord blood collection
Data confirm that delaying cord clamping for up to 1 minute does not significantly diminish the cell count of cord blood collected for preservation.14 We want families who opt for delayed clamping to know that they can still choose to preserve newborn stem cells.
Evidence suggests that cryopreserved newborn stem cells can be preserved indefinitely
Stem cells stored in proper cryogenic storage are believed to remain viable for decades and likely indefinitely, ensuring their potential use for years to come.15 Informed parents who choose to preserve their family’s newborn cells now could be preserving a resource to be used for generations to come, not only for the established and investigational uses available today, but for the potential uses available in the future.
Help us spread the word to give more families access to the power of newborn stem cells
The ability to harness the current and potential power of newborn stem cells is largely dependent upon the patient’s decision to preserve their baby’s stem cells. This decision is predicated upon patient awareness of the advantages and applications of these cells, information and guidance that is provided in large part by their healthcare providers. With a focus on affordability, Insception Lifebank wants this valuable resource to be available to everyone, which is why we offer payment plans and discounts. We recommend these essential conversations about newborn stem cell preservation begin at the confirmation of pregnancy, with continued discussion with patients around 28 weeks of pregnancy.
Stem cell educational resources for providers and patients
We understand it’s important to have access to reliable and quality educational resources for both providers and their patients, and we’ve made it easy to access information about stem cell science and options for newborn stem cell preservation:
- Insception Lifebank – Both you and your patients have unlimited access to our Cord Blood Clinical Specialists, Insception Lifebank Account Managers, and up-to-date educational materials.
- Parent’s Guide to Cord Blood Foundation – a nonprofit organization aimed at educating parents about the most accurate and updated information on newborn stem cells.
Celebrate Cord Blood Awareness Month by spreading the word!
Be an advocate for patient awareness and help us spread the word about the exciting current and potential benefits of newborn stem cells in transplant and regenerative medicine.
1. Zhu, X., Tang, B. & Sun, Z. Umbilical cord blood transplantation: Still growing and improving. Stem Cells Transl. Med.10, S62–S74 (2021). 2. Mayani, H., Wagner, J. E. & Broxmeyer, H. E. Cord blood research, banking, and transplantation: achievements, challenges, and perspectives. Bone Marrow Transplant.55, 48–61 (2020). 3. Wagner JE. Cord blood 2.0: state of the art and future directions in transplant medicine. Blood Res. 2019 Mar;54(1):7-9. doi: 10.5045/br.2019.54.1.7. Epub 2019 Mar 21. PMID: 30956957; PMCID: PMC6439299. 4. Baraniak, P. R. & McDevitt, T. C. Stem cell paracrine actions and tissue regeneration. Regen. Med.5, 121–143 (2009). 5. U.S National Library of Medicine. ClinicalTrials.gov. Accessed October 21, 2022. https://clinicaltrials.gov/ 6. Couto PS, Bersenev A, Verter F. The first decade of advanced cell therapy clinical trials using perinatal cells (2005/2015). Regenerative Medicine. 2017;12(8):953-968. doi:10.2217/rme-2017-0066 7. Torre P, Flores AI. Current Status and Future Prospects of Perinatal Stem Cells. Genes (Basel). 2020 Dec 23;12(1):6. doi: 10.3390/genes12010006. PMID: 33374593; PMCID: PMC7822425. 8. Regenerative Medicine. AABB. Retrieved from: https://www.aabb.org/news-resources/resources/cellular-therapies/facts-about-cellular-therapies/regenerative-medicine. 9. Kindwall-Keller TL, Ballen KK. Umbilical cord blood: The promise and the uncertainty. Stem Cells Transl Med. 2020;9(10):1153-1162. 10. Pan, K., Farrukh, H., Chittepu, V.C.S.R. et al. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res 41, 119 (2022). https://doi.org/10.1186/s13046-022-02327-z. 11. Liu E, Marin D, Banerjee P, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med. 2020;382(6):545-553. doi:10.1056/NEJMoa1910607. 12. Shah N, Li L, McCarty J, et al. Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br J Haematol. 2017;177(3):457-466. doi:10.1111/bjh.14570. 13. Sagoo, P., Gaspar, H.B. The transformative potential of HSC gene therapy as a genetic medicine. Gene Ther (2021). https://doi.org/10.1038/s41434-021-00261-x 14. Ciubotariu R, Scaradavou A, Ciubotariu I, Tarnawski M, Lloyd S, Albano M, Dobrila L, Rubinstein P, Grunebaum A. Impact of delayed umbilical cord clamping on public cord blood donations: can we help future patients and benefit infant donors? Transfusion. 2018 Jun;58(6):1427-1433. doi: 10.1111/trf.14574. Epub 2018 Mar 25. PMID: 29574750. 15. Broxmeyer HE, Lee MR, Hangoc G, et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood. 2011;117(18):4773-7.
 Sign in
Sign in 